In this post, we discuss modern flow cytometry as a game-changer for cell functional assays, immune monitoring, and function-based screening of drug candidates to support and accelerate your biomarker strategy and translational programs.
The Importance of Biomarkers in Drug Discovery and Development
Flow cytometry is used to the effects of drug candidates on intended biological pathways by accurately measuring biomarkers which serve as predictive surrogates of efficacy.
In addition, modern flow cytometry is being used to develop precision medicine approaches given that many diseases have highly heterogeneous biology that results in individual patients showing a spectrum of responses to the same treatment.
The high heterogeneity of many diseases explains, in part, the high failure rate of drugs in clinical development. Only ~0.1% of new drug candidates survive the journey from preclinical research to marketing approval.
To improve on this dismal statistic, there has been a call for better assays and tools to evaluate and characterize drug candidates, and flow cytometry represents such a tool.
Further, it can be applied to many different research models, which can help determine a candidate’s chances for success given the golden rule that “the more preclinical models that your candidate succeeds in, the less chances that your candidate will fail in the later stages of drug discovery and development.”
Researchers across indications such as oncology, cancer immunology, inflammatory and autoimmune diseases, are extensively using biomarker strategies to improve their drug discovery and clinical success.
Furthermore, incorporating biomarkers into the drug discovery process can provide a better understanding of the disease during target discovery, since they allow drug activity and safety to be measured using an endpoint related to the mechanism-of-action of the drug.
The Value of Flow Cytometry for Immune Monitoring and Cell Functional Assays
Flow cytometry is a versatile tool that is increasingly being used as drugs targeting the immune system become more common. Flow cytometry allows dissecting the impact of your drug candidates on cells, literary, inside-out. A good example is assessing T cell exhaustion (Tex) by examining surface co-inhibitory molecules and Tex transcription factors via intracellular staining.
Flow cytometry is commonly used for immune monitoring and cell functional assays in preclinical in vitro and in vivo models, primarily because immune cell subsets, cytokine production profiles, and activation status can be characterized from large and complex cell mixtures.
By analyzing these parameters, researchers can better understand the role of the immune system in disease, which is a major factor in developing novel effective and safe therapies across a range of therapeutic areas, including immuno-oncology, inflammatory, and autoimmune diseases.
Modern flow cytometry techniques have been a game-changer due to their ability to accommodate high-throughput and multiparameter assays with high precision and high reproducibility.
Flow cytometry is a comprehensive tool for investigating drug mechanism-of-action and pharmacodynamics through immune cell monitoring and functional assessment.
Modern high-throughput and multiparameter flow cytometry techniques are commonly used to:
- Analyze expression of cell surface and intracellular molecules
- Analyze cell size and volume at the single cell level
- Characterize and define different cell types in a heterogeneous cell population
- Quantify therapeutic effect by assessing frequencies of immune cell subsets
A variety of commercial technologies are available to support studies aimed at characterizing complex immune-phenotypes and rare cells of interest from samples with limited availability, including (among others):
- Multi-color analyses and/or sorting: BD FACSCalibur™, BD Accuri™ C6, BD LSRFortessa™
- Semi-automated dissociation of tissues into single-cell suspensions or homogenates: gentleMACS™ Dissociator (Miltenyi Biotec, Inc.)
Since flow cytometry is a single cell-based assay, a recognized limitation of this technology is that it can sometimes be challenging to obtain single cell populations from solid tissues.
Often, this results in clusters/clumps of cells, and overall low cell viability.
Sensitivity can also be an issue when it comes to very rare cell populations, although other technologies, such as “Chipcytometry” (an imaging cytometry platform enabling ultra-deep phenotyping) and “Rarecyte” can help overcome this problem by serving as an integrated rare cell analysis platform for tissue samples and cell suspensions.
| |
Platforms |
Applicatons & comments |
Single cell based |
Fortessa, etc., |
Multi-color: surface, intracellular |
| Chipcytometry |
Multi-color and location |
| Rarecyte |
Detect/quanitfy rare immune cells |
The following sections describe how flow cytometry can be used to support drug development in cancer immunology, inflammation, and autoimmune disease.
Flow Cytometry for Drug Development in Cancer Immunology Studies
Cancer immunology studies are focused on how immune cells respond to a cancer. In general, researchers are interested in identifying and validating predictive biomarkers for guiding treatment decisions, and to discover novel therapies, and signatures associated with off-target responses in patients.
Flow cytometry has been successfully used to support immuno-oncology studies since it is a powerful tool to assess single cell immune profiles in drug development.
It has also been used to develop therapeutic cell therapies (e.g., CAR-T, CAR-NK) since it can enrich for cells of interest and isolate positive edits quickly and efficiently.
A major benefit of flow cytometry is its flexibility (as compared to other methods like histology, IHC, WB, ELISA, MSD, ELISPOT), which allows researchers to conduct a cascade of assays with very small sample volumes.
For example, cell suspensions prepared from tumor samples can be used for immunophenotyping, MDSC and M1/M2 macrophages, and co-inhibitory, co-stimulatory and T cell exhaustion biomarkers (surface or intracellular staining), and cell functional assays.
Genomic material (RNA or DNA) can also be isolated and used for downstream studies, such as a mouse I/O RNA-Seq panel, to gain a thorough understanding of the genes linked with tumor immunity in your studies.
Flow cytometry can be used to support preclinical cancer immunology studies (in syngeneic mouse models, PDX models, patient-derived tumor organoids, etc.) by analyzing:
- Tumor deposits
- Tumor Infiltrating Lymphocytes (TILs)
- Draining lymph nodes, spleen, peripheral blood
- T cell Exhaustion Markers
- Myeloid Derived Suppressor Cells (MDSC) and M1/M2 Macrophages
- Cell Functional Assays
- Antibody Depend Cell Cytotoxicity
| Marker |
Immune Cell Population |
| CD45 |
Total leukocytes |
| CD3 |
Total T cells |
| CD4 |
CD4+ T helper cells |
| CD8 |
CD8+ Cytotoxic T cells |
| ^CD44/CD62L |
Naïve, Memory and Effector T Cell |
| ^CD69/CD44/OX40/CD25 etc |
Activation Markers |
| CD4+CD25+FoxP3+ |
Regulatory T cells |
| CD11b+IA/IElow/-Ly6c/Ly6g |
G-MDSC and M-MDSC |
| CD11b+ F4/80 |
Macrophages |
| IA/IE/CD11c/CD206 |
M1 and M2 Macrophages |
| CD3-CD335+ |
NK cells |
| CD3+CD335+ |
NKT cells |
| CD19 |
B cells |
| ^TNF-a/IFN-r/IL-7/IL-3 etc |
Cytokines |
| ^PD-1/PD-L1/CTLA4/TIM-3 etc |
Check Point Inhibitors |
| ^Granzym B etc |
Commonly request markers |
| K167/Brd U/PNCA etc |
Proliferation |
| Live/Dead (fixable) |
Live/Dead |
Note: ^Markers can be added by client's request
Examples of Immunophenotyping and Biomarkers of Murine IO and TILs
Flow Cytometry for Drug Development in Inflammation and Autoimmune studies
Flow cytometry is commonly used for assessing the phenotype and functionality of immune cell populations in inflammation and autoimmunity preclinical models, including inflammatory bowel disease in vivo models.
Flow cytometry is routinely used for immunophenotyping of bronchoalveolar lavage fluid, synovial fluid, gut, and lymph nodes, and can be used in in vivo models of inflammation and autoimmunity for measuring the impact of therapeutics on immune cells and their subtypes.
We can use this method to support inflammation and autoimmunity studies by analyzing TH1, Th2, TH17, M1/M2 macrophages, regulatory T cells, and myeloid derived suppressor cells (MDSC) isolated from colon and mesenteric lymph nodes, spleen, and peripheral blood from a wide range of preclinical models including: Inflammatory bowel disease (IBD), Graft-versus-host-disease (GVHD), Non-alcoholic steatohepatitis (NASH), Multiple sclerosis (MS), Lupus, Rheumatoid arthritis (RA), Parkinson’s Disease, Type-2 Diabetes, and Psoriasis.
In vitro PBMC Assays to Identify Immunomodulatory Drug Candidates
In vitro peripheral mononuclear cell (PBMC)-based assays, which make use of flow cytometry, are often used for functional characterization of immunomodulatory candidate drugs.
Below we will discuss the recall antigen assay and tumor organoid co-culture assays.
The most commonly used PBMC-based assay is the “recall antigen assay.” This assay employs whole PBMC from characterized donors that are stimulated with mitogens to provide a functional readout for screening and characterizing immunomodulatory drug candidates. Alternatively, PBMC may be stimulated with mitogens, polyclonal activators such as PHA, anti-CD3/anti-CD28, Staphylococcal enterotoxin B, Con-A, or in a mixed lymphocyte response setting. The impact of immunomodulatory drug candidates may be assessed in such settings.
Recall antigen assays have played an important role in the development of approved immune checkpoint inhibitors since they were key for demonstrating the functional activity of specific clones or lead drugs.
While there are several limitations associated with the traditional recall antigen assay, more recently, improved protocols have been developed, including an assay that involves spiking with extracellular adenosine to recapitulate some aspects of the suppressed tumor microenvironment.
For instance, Kleiman et al. (2021) adopted a flow cytometry-based tetramer readout to measure CD8+ antigen-specific T cell expansion seven days post-peptide stimulation (see illustration below).
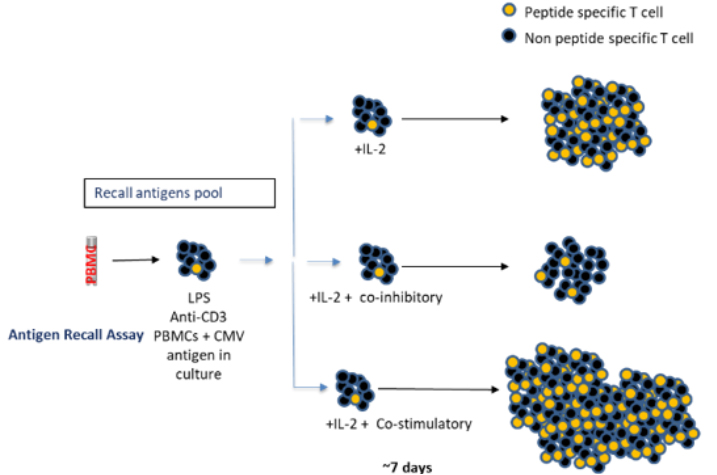
Adapted from Kleiman E. et al. (2021). Adenosine-related small molecules show utility of recall antigen assay to screen compounds for off-target effects on memory T cells. Sci Rep 11, 9561 (2021). https://doi.org/10.1038/s41598-021-88965-3. Used under Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Surrogate of immune-activation for screening T cell modifiers. There are multiple surrogates of activated T cells, intracellular staining for cytokines or activation markers such as KI67, or surface markers such as the expression of 41BB (CD137), CD25, CD69, and MHC Class II.
Below is an example of such studies where the expression of CD137 is correlated with peptide specific MHC Tetramer positive CD8 T cells.
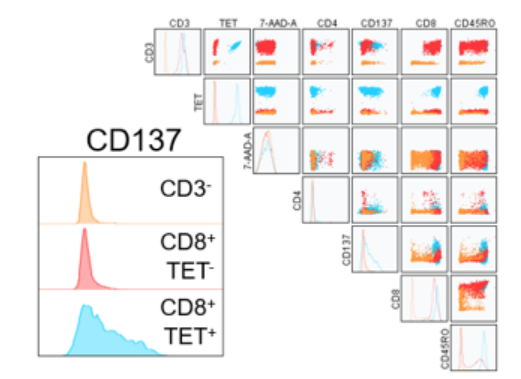
CD137 co-stimulatory surface marker used as a surrogate for activation status on day 7 of recall. Displayed is data from HLA-A*02:01. Donor #1 PBMCs treated with peptide only (NLVPMVATV) as a representative. CD137 expression non-overlayed histograms (bottom left) for CD3 negative cells (orange), CD3+CD8+ tetramer negative cells (red) or CD3+CD8+ tetramer positive cells (blue). Overlay plots include CD3, tetramer, 7AAD viability dye, CD4, CD8, and CD45RO.
Organoid co-cultures offer a simple and flexible in vitro system for reconstituting complex biological systems that allows for the study of each of the components.
For instance, co-culturing tumor organoids with non-autologous PBMCs from healthy donors can capture important aspects of the human tumor microenvironment, including different cellular components that are not easy to recapitulate using other systems.
These co-cultures allow for modeling complex and dynamic interactions between the tumor and the immune cells in vitro, which have traditionally been investigated only using in vivo complex humanized systems. The following graphic depicts an experiment that used flow cytometry to assess tumor organoid killing by T cells from non-autologous PBMCs.
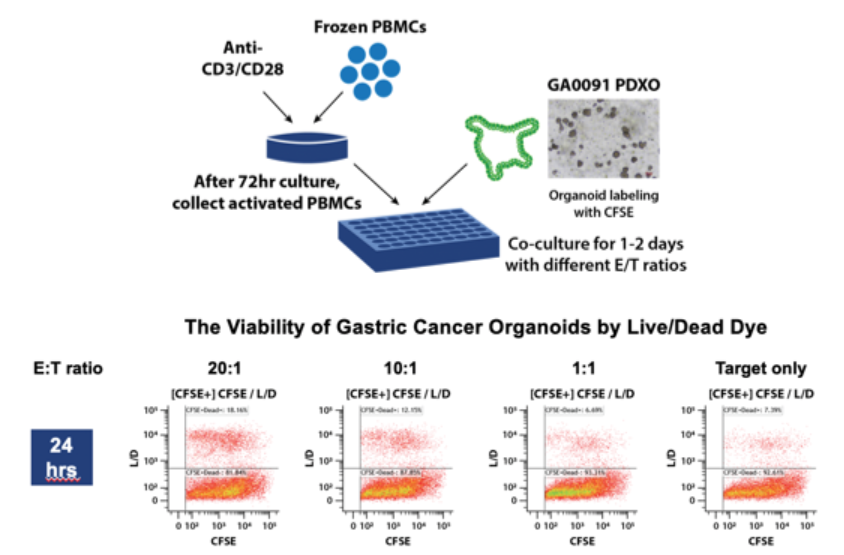
Flow cytometry-based tumor organoid killing by T cells from non-autologous PBMCs. PBMCs from a healthy donor were pre-activated by anti-CD3/anti-CD28 for 3 days and co-cultured with CFSE-labeled tumor organoids for 1-2 days. The viability of organoid cells was evaluated by flow cytometry after dissociation into single cell suspension.
Conclusion
Biomarker strategies are being incorporated earlier in the drug development process since they can provide invaluable information for understanding mechanism-of-action of a novel therapeutic, and they can guide downstream development decisions in a data-driven manner.
Flow cytometry is a highly flexible tool that is integral for immune cell monitoring and functional characterization of these cells.
Further, modern flow cytometry tools are amenable to high-throughput and multiparameter assays, and it has been a game-changer for assessing investigational agents targeting the immune system across a range of therapeutic areas.
Crown Bioscience has extensive experience in the use of flow cytometry for supporting preclinical biomarker programs for drug discovery and development across a variety of disease indications. Our professional flow cytometry services are designed to help companies accelerate their drug development programs. Please visit our flow cytometry site to learn more about our expertise in this area.