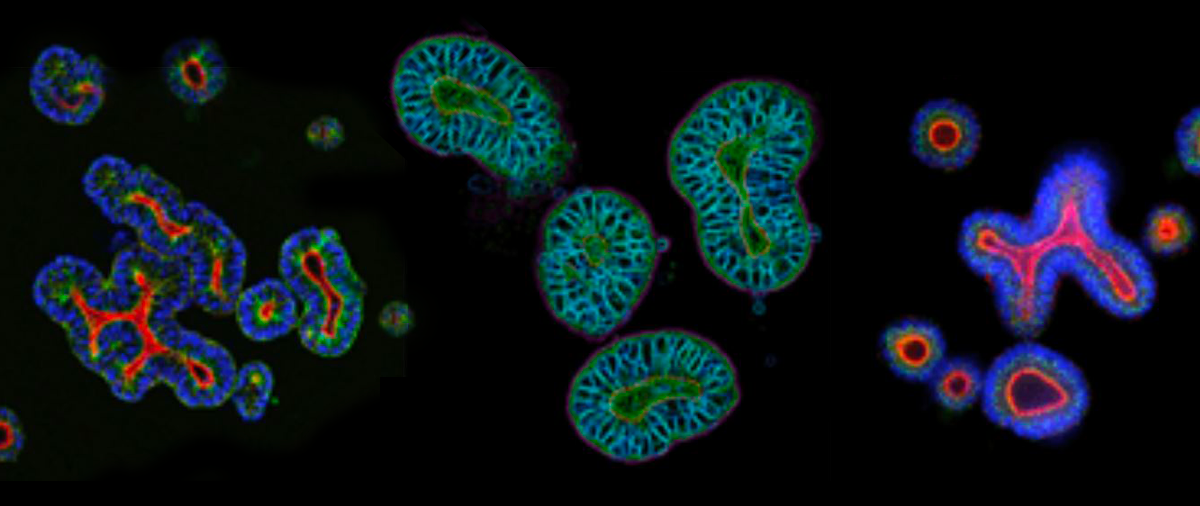
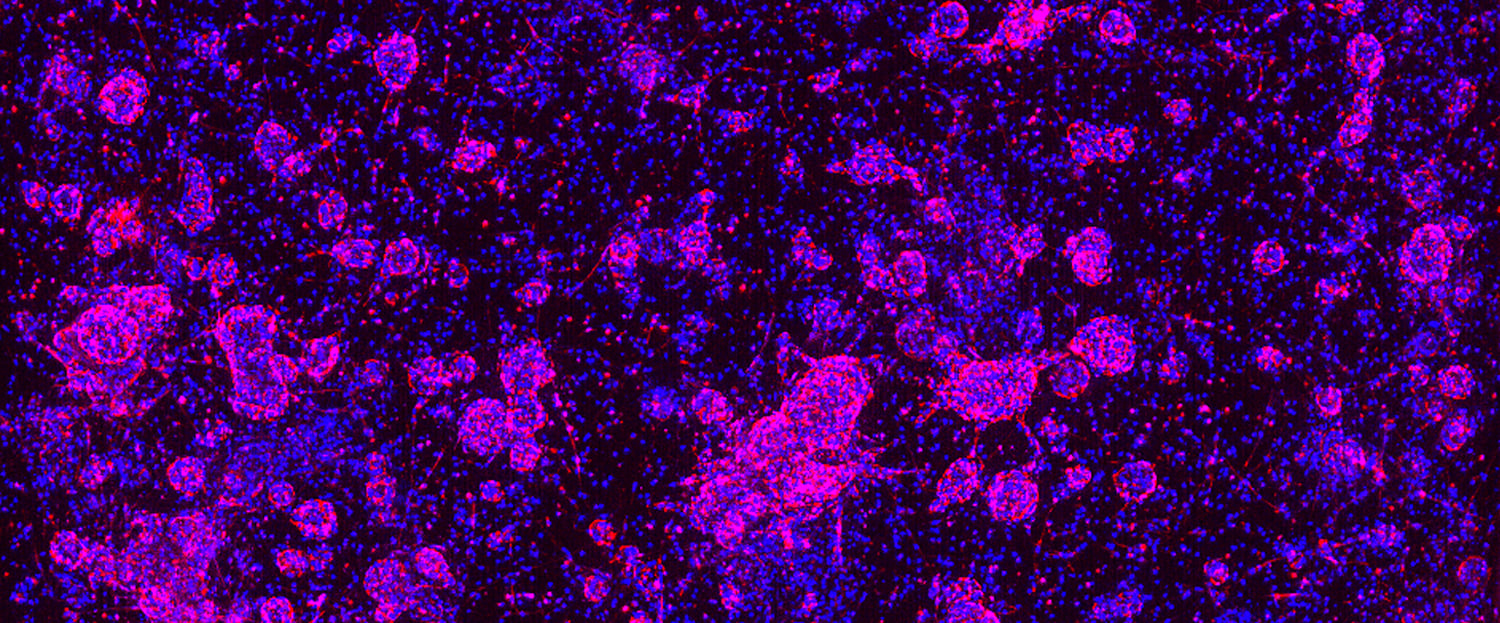
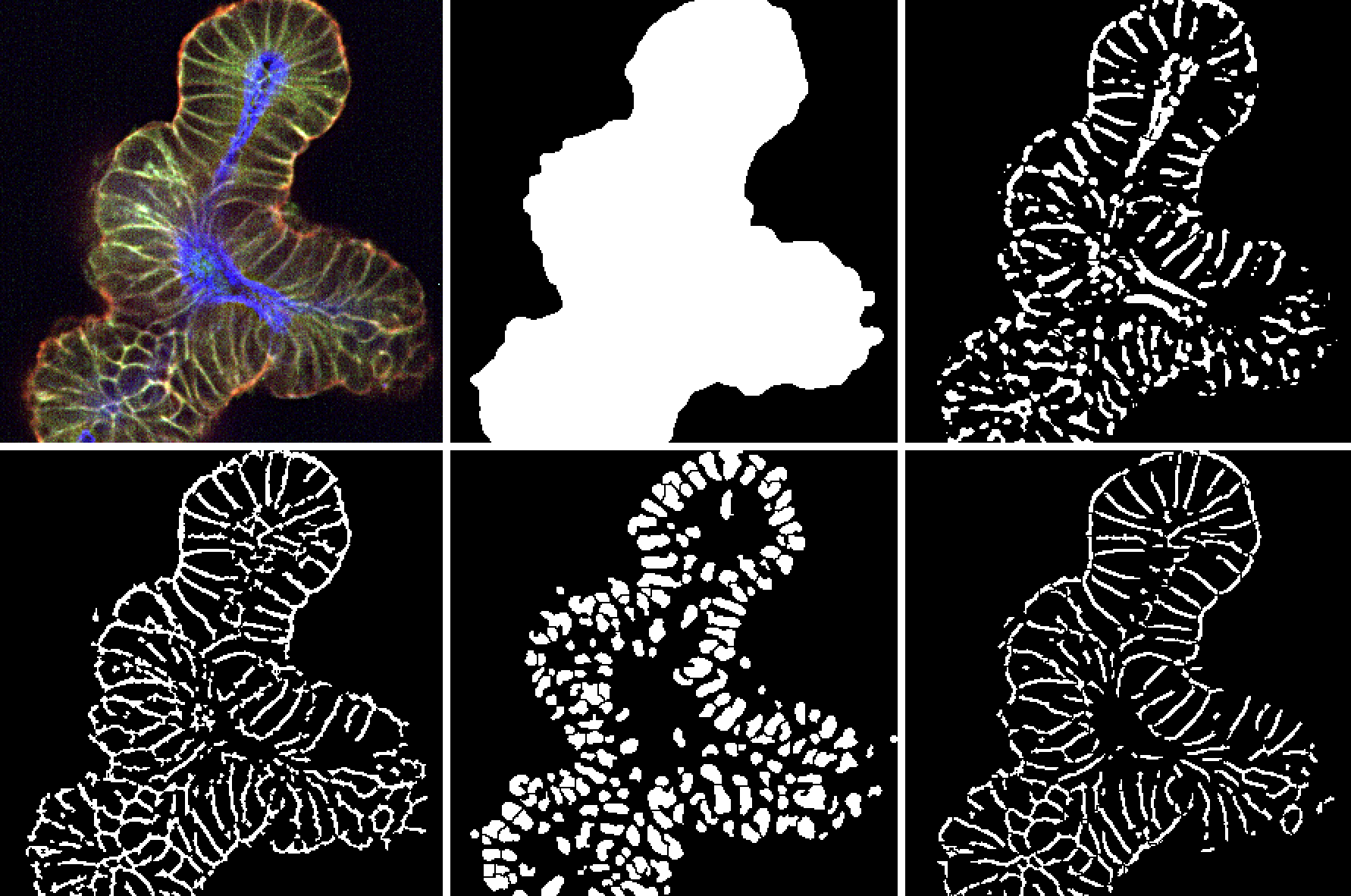
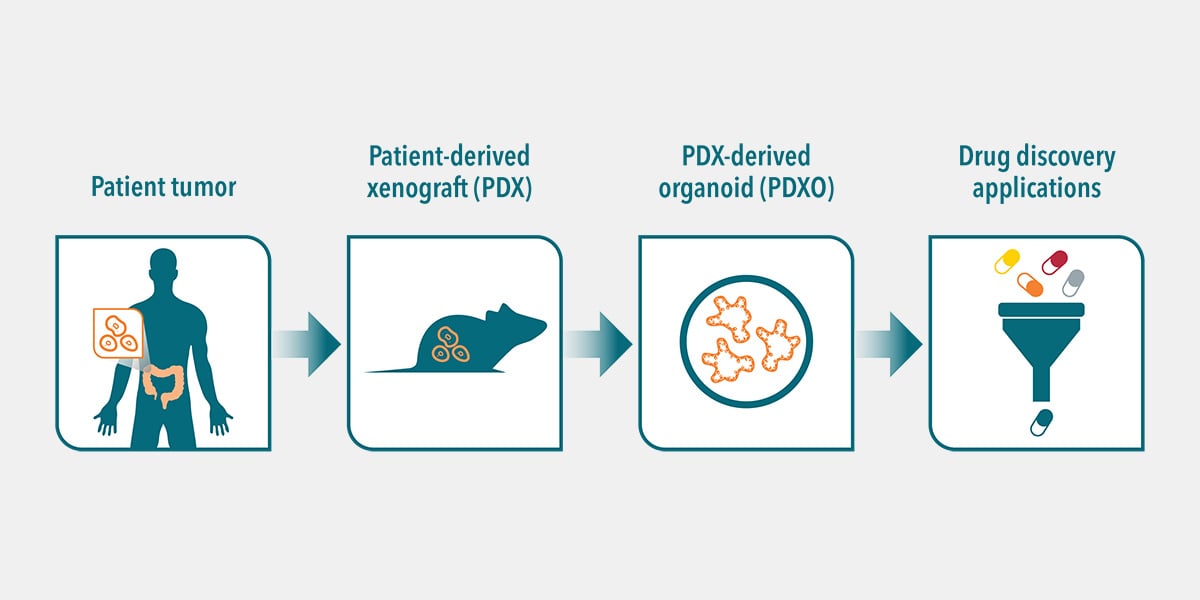
The use of more clinically relevant preclinical models can help to reduce anticancer drug attrition rates by providing a better understanding of the safety, efficacy, and mechanism of a drug candidate, which can help inform downstream decisions.
This blog post explores the value of adult stem cell-derived 3D in vitro tumor organoids in drug discovery, particularly in identifying anticancer agents with improved translational potential, as highlighted in recent peer-reviewed publications.
What Is an Organoid?
The term "organoid" has previously been used when referring to groups of cells that create 3D structures when grown without attachment substrates.
Today, it specifically refers to a 3D structure with cells that establish or retain the identity of the organ/tissue being modeled, both stem cells and their progeny. Organoids are typically derived from either pluripotent stem cells (PSCs), i.e., embryonic stem cells or induced PSCs, or multipotent organ-specific adult stem cells (ASCs).
Organoids are composed of multiple cell types found in the specific parental organ/tissue (which can be either healthy or diseased, such as tumor tissue), exhibit some aspect of its functional properties, and self-organize following the same intrinsic principles as the original organ/tissue.
Organoids are distinct from other 3D models. For example, spheroids are created from a single cell type or a mixture of cells and can originate from stem cells, primary cells, immortalized cell lines, or human tissue. They are also composed of various layers of cells, which can result in heterogeneity, including cells that are oxygenated, hypoxic, proliferating, nonproliferating, and/or necrotic.
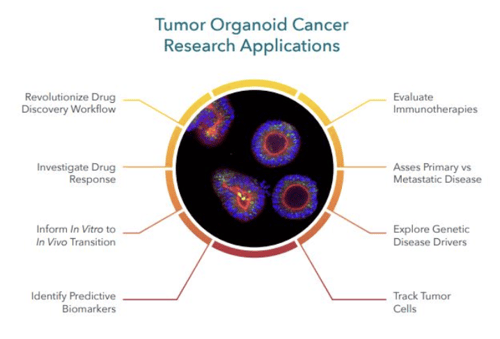
Key Differences Between PSC- and ASC-Derived Organoids
PSC organoids are often used to model organogenesis. However, the protocols to generate them are more time consuming and complex compared to those for developing ASC-based organoids (see our previous blog post for a more in-depth discussion).
In short, ASC-derived organoids circumvent some of the steps required for PSC organoids because they are established from tissue-specific stem cell populations using optimized protocols.
Robust protocols use optimized conditions to preserve the stem cell self-renewing and differentiating potential of purified single ASCs or tissue-specific microstructures. The resulting organoids faithfully recapitulate the phenotypic and genetic features of their original tissue.
Based on data from published studies (for example, see here, here and here), ASC-derived organoids represent the first clinically relevant 3D in vitro model to show the following:
- Genomic and phenotypic stability in long-term culture and after cryopreservation,
- Ease of scalability relative to in vivo models, and
- Clinical predictivity.
Furthermore, their genomic and phenotypic stability after cryopreservation has allowed the development of living organoid biobanks (such as OrganoidBase™) containing models covering multiple solid cancer types and enabled clinical trials as surrogate subjects in vivo. This approach is the equivalent of a clinical trial but carried out in a dish, expediting drug development, and reducing the high attrition rate of oncology candidates.
The Value of ASC-Derived Organoids: Scalability, Reproducibility, and Clinical Relevance
Adult stem cell-derived in vitro tumor organoids are powerful predictive preclinical models for cancer drug discovery and development largely due to characteristics that enable their scalability, reliability, and high patient predictivity. Below are summaries of two recent studies that highlight the power and potential of tumor organoids for cancer drug discovery and development.
In a study published in PLOS ONE, scientists from Crown Bioscience reported on the development of a biobank of patient-derived-xenograft organoids (PDXOs) from a PDX library. The goal was to assess the biological equivalency or interchangeability between the PDXs and corresponding PDXOs and compare their pharmacological responses.
The scientists tested more than 550 matched in vitro and in vivo models representing a variety of cancer types. Overall, the biological equivalency between the two types of models was confirmed using extensive genomic and histopathological characterization. The in vitro PDXO and in vivo PDX pharmacology had high correlation.
The biobank was also successfully used for high-throughput “matrix” screening for both lead compounds and indications, immune cell co-cultures for immuno-therapies, and genetic engineering for in vitro/in vivo imaging. Overall, it was concluded:
“this large biobank of >550 matched pairs of PDXs/PDXOs across different cancers could become a powerful tool for the future of cancer drug discovery.”
A second study in Nature Cancer reported preclinical data on a bispecific antibody that was shown to prevent the onset of metastasis and slow the growth of primary tumors in experimental models.
A large-scale functional screen of dual-targeting bispecific antibodies (bAbs) on a heterogeneous colorectal cancer PDO biobank (developed using optimized protocols) and paired healthy colonic mucosa samples was conducted.
Overall, 500+ bAbs generated against Wingless-related integration site (WNT) and receptor tyrosine kinase targets were functionally evaluated by high-content imaging to capture the complexity of PDO responses. This enabled discriminating antibodies that were active against a broad range of mutational profiles and tumor subtypes, with hit validation and mechanism-of-action studies that enabled selection of optimally performing antibodies.
This strategy resulted in identifying MCLA-158, a bAb that triggers epidermal growth factor receptor degradation in leucine-rich repeat-containing G-protein-coupled receptor 5-positive (LGR5+) cancer stem cells but with minimal toxicity toward healthy LGR5+ colon stem cells.
MCLA-158 therapeutic properties (i.e., growth inhibition of KRAS-mutant colorectal cancers, blockade of metastasis initiation, and suppression of tumor outgrowth) were confirmed in matched PDX models for several epithelial cancer types.
This groundbreaking work confirmed the power of 3D in vitro tumor organoids by shrinking the time between initial discovery to clinical trials to approximately five years.
Conclusion
Organoids are becoming increasingly popular in cancer drug discovery and development since they offer a balance between scalability and clinical-predictivity compared to in vivo and other in vitro models. These models accurately reflect the genomic, morphological, and pathophysiological features of the original tumor. Overall, organoids have great potential for improving the efficiency and success of cancer drug discovery, and are a promising avenue for future research.
Visit Crown Bioscience’s Organoids for Oncology Drug Development site to learn more about how we help researchers advance their drug discovery and development programs.