 Review how, and why, to use tumor organoids in in vitro oncology drug screening programs.
Review how, and why, to use tumor organoids in in vitro oncology drug screening programs.
Why Use Organoids for Oncology Drug Screening?
Before we jump straight into how to use tumor organoids for drug screening, one key question to answer is why use organoids – why not continue with the standard in vitro drug screening platforms I’m using now?
The main answer is clinical relevance. Typical drug discovery relies heavily on immortalized cell lines for high throughput screening (HTS), testing both target engagement and cellular activity. While there are many advantages to this approach, continuous maintenance on plastic results in cell line genetic drift, poor translatability, and reduced disease relevance. This all contributes to the high attrition rate currently seen in oncology drug development.
While more predictive models have been developed using clinical material, such as primary cultures ex vivo or patient-derived xenografts (PDX) in vivo, these haven’t been amenable to HTS. Primary cultures are limited by the amount of tissue available and expansion for follow up studies.
PDX produce abundant material in vivo and provide a highly translational platform for late stage drug development, including through mouse clinical trials (MCT). However, the time and cost of PDX development is prohibitive and these models haven’t back translated effectively for high-throughput in vitro use.
Early stage robust and scalable screening platforms are now critically needed to provide clinically-relevant and translatable data to guide drug development, patient selection, and companion diagnostic (CDx) development as early as possible. Organoids have the potential to fill this gap and provide the in vitro preclinical model needed to overcome current screening challenges.
Tumor Organoids
We’ve written extensively in previous posts on the background and development of organoids and tumor organoids. Briefly, patient-derived organoids are generated in vitro from stem cells or progenitor cells grown in 3D. Organoids have the ability to self-organize and self-renew, resulting in the development of “mini-organs” in a dish. These organoids contain multiple differentiated cell lineages exhibiting remarkable similarities to the in vivo organ of origin.
Pioneering work from Hubrecht Organoid Technology (HUB) has generated robust protocols to develop organoids from adult stem cells from both normal and diseased tissue. HUB tumor organoids recapitulate the genomic, morphological, and pathophysiological characteristics of their parental tumor. This provides highly clinically relevant 3D in vitro tumor models for drug discovery.
Tumor organoids are cryopreserved to generate biobanks of models, as well as expanded following resuscitation without losing the organoid’s original identity. This means organoids are a great tool for understanding molecular mechanisms of cancer as well as the development of novel therapeutics. Tumor organoids are also predictive of patient treatment response, therefore providing in vitro models which are much more relevant than standard 2D in vitro platforms.
Drug Screening Using Tumor Organoids
Building the Largest Tumor Organoid Collection to Represent Patient Diversity
To start using tumor organoids for oncology drug screening, the first step is to develop a large panel of organoids covering a wide spectrum of cancer types, similar to the large panels of cell lines currently available for HTS or PDX collections for mouse clinical trials.
Historically, tumor organoids have been derived directly from patient tumors (patient-derived organoids or PDO). While this is still a highly valid technique producing very relevant models for screening, developing the panel of models needed can be time consuming, due to having to source patient tumor tissue from a range of different cancers.
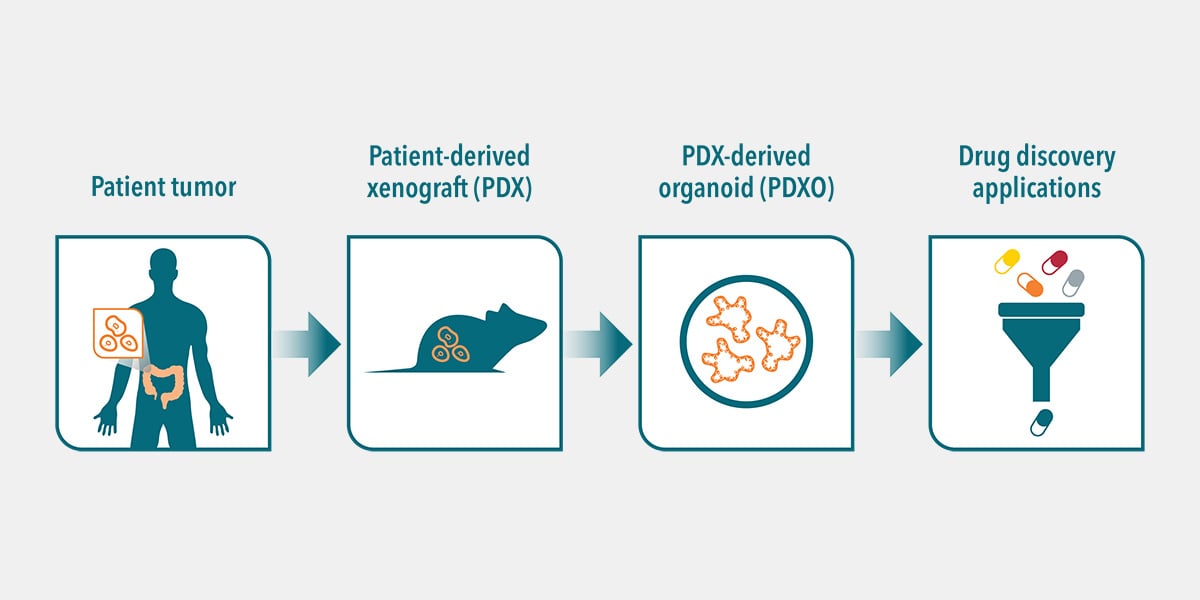
A new approach is to develop PDX-derived organoids (PDXO), from the large collections of in vivo PDX models that are already available and well annotated. This offers a unique opportunity to expand the repertoire of patient-derived organoid models available, including a wider range of cancer indications with a spectrum of mutational profiles and pharmacological response. This includes poorly responsive or resistant tumors.
PDXO are established from the cancer stem cells found within PDX tumors, in the same way that PDO are derived from patient tumors. The developed PDXO and parental PDX show biological equivalence, based on their shared genomic diversity, tumor heterogeneity, histopathology, and response to drugs. PDXOs also maintain stable genomic, morphological, and pathophysiological identities of the corresponding PDX tumor over many passages.
This therefore highlights PDXO as a unique in vitro platform featuring the gold standard patient relevance of PDX models, the scalability of conventional patient-derived organoids, combined with developmental efficiency from the collections of thousands of PDX already generated. This combination provides an ideal platform for large scale drug screens in patient-relevant models, including combination strategy testing, biomarker identification, and CDx development.
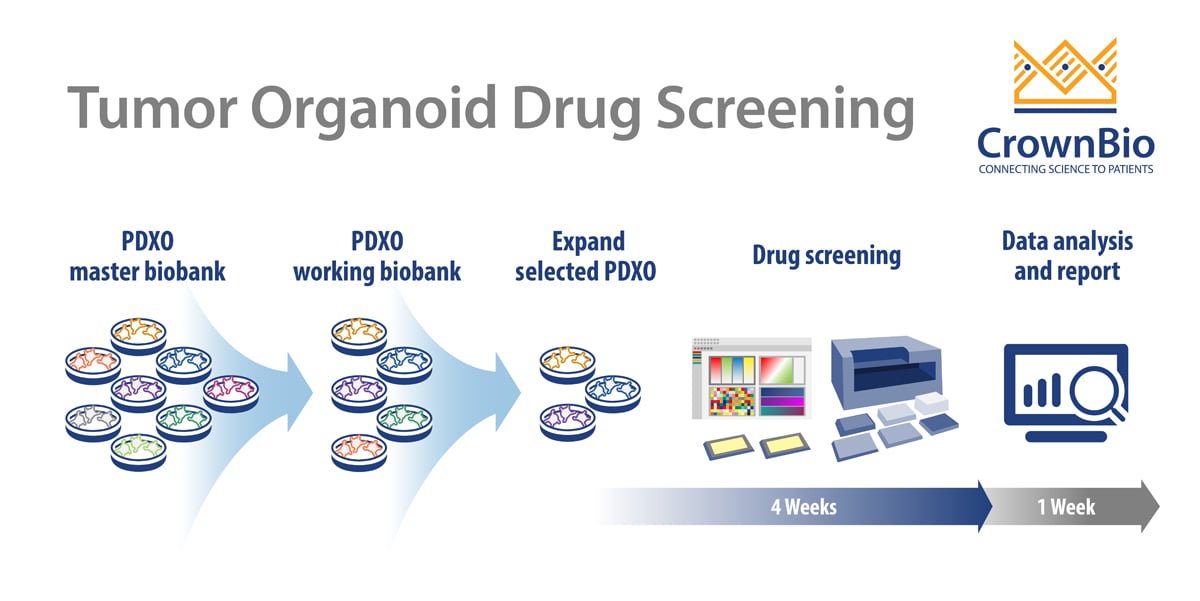
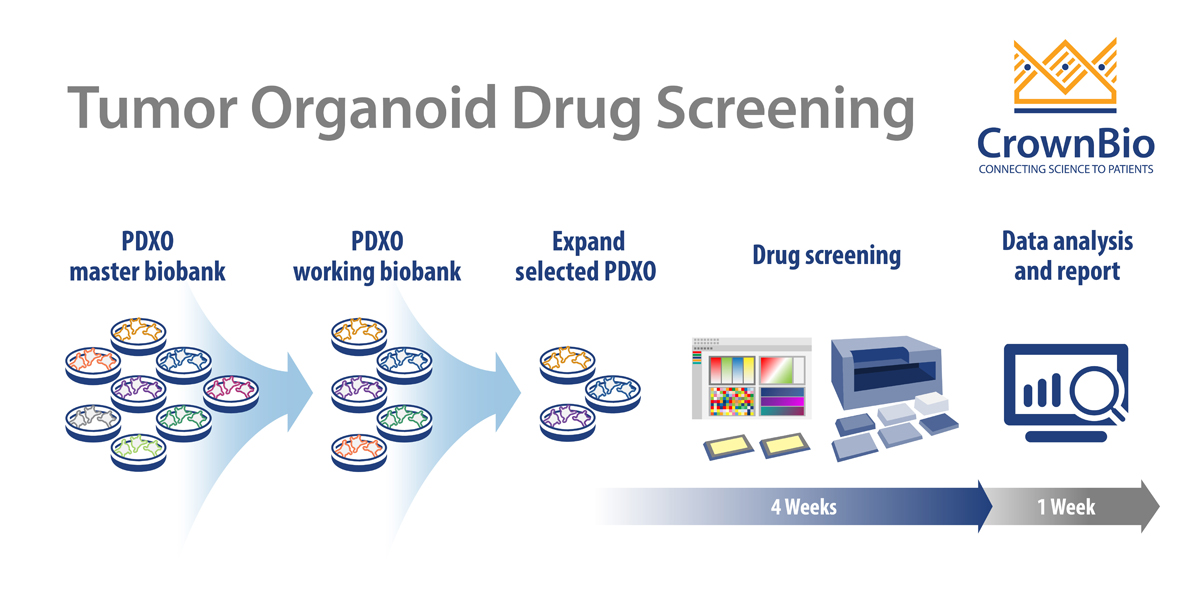
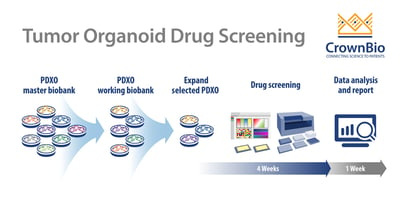
PDXO Screen Workflow
Like other typical HTS screening, PDXO drug screening is an automated, highly reproducible, and robust system, aiming to improve efficiency. The added benefit with PDXO screening is being highly predictive in early stage drug development.
Starting with the master biobank of cryopreserved models, PDXO are expanded into a working biobank, ready for HTS. These PDXO biobanks are mycoplasma tested and STR profiled with sufficient banking, resuscitation, and growth profiling performed to enable repeat use of models. Specific organoids can be selected from an online organoid biobank database. Selected PDXO are then expanded as required and seeded into multi-well plates for drug screening.
Screens are run in a similar way to a 2D screen:
- In a 384-well format
- Typically generating IC50 values for 8 compounds
- With positive and negative controls included on each plate.
Test agents are incubated with the organoids for 5 days with a CellTiter-Glo® viability readout or morphology assessment as endpoints. Model expansion and enrollment into the study to IC50 readout and analysis can be as quick as 5 weeks for multiple agents and multiple tumor models. Endpoint readouts can be extended to other physiological parameters, such as apoptosis or high content imaging.
Key Benefits of Tumor Organoids in Drug Screens
There are many benefits to using organoids in general in oncology drug screens, some of which were discussed above. This includes organoids providing increased patient relevance due to preserving original and phenotypic features as well as tumor 3D architecture, all of which are known to influence drug response.
Tumor organoid drug screens also offer high quality readouts, for example, high signal to noise ratio and low intra-plate variation demonstrating the robustness of the platform.
PDXO screening enables a broader evaluation of compounds and their combinations over a large collection of tumor models. Studies can then move directly to in vivo modeling using matched PDX models.
Differences Between Tumor Organoid and Standard 2D Drug Screens
There are some differences from standard 2D screens that need to be considered when planning an organoid screen.
As discussed above, tumor organoids can be cryopreserved then resuscitated for HTS enrolment. This can result in a lead in time slightly longer than standard in vitro cell lines.
The current 384-format for organoid screening is also not as high throughput as standard cell line HTS. This is partly due to the expansion potential of organoids as the culturing and plating criteria for organoids is different to 2D monolayer cultures. For example, the size, density, and morphology of organoids in 3D culture and passaging steps require slightly more time.
Experience and expertise in organoid culturing is therefore a necessity when designing screening protocols as well as genomic, histopathology, and pharmacology profiling of tumor organoids.
Based on the clinical relevance of tumor organoids, it’s clear that organoid screening data will be unmatched by other in vitro screening tools. The availability of such large scale screens are exciting times in early drug discovery.
Conclusion
A lack of preclinical model systems recapitulating primary patient tumors is a major setback in early stage oncology drug development. The use of in vitro tumor organoids in large-scale drug screens can help overcome this providing a robust, scalable, and predictive 3D system to reinvigorate current development workflows.