Combination strategies of irradiation and immunotherapy have been shown to harness the immune system to improve responses in difficult-to-treat and/or unresponsive tumors. But, determining the administration sequence of radiotherapy and immunotherapy is not straightforward, so as a result, preclinical studies are being used to pinpoint the precise sequence. In this blog, we look at how preclinical studies can progress irradiation and immunotherapy combination regimens.
The Importance of Combining Radiotherapy and Immunotherapy
The complexity of cancer as a dynamic and highly heterogeneous disease motivates researchers to develop novel treatment paradigms that can effectively manage metastatic disease, reduce cancer recurrence, and improve survival rates.
Radiotherapy has been used for decades to manage local tumor growth. Used alone, it rarely induces an abscopal response, also known as a systemic antitumor immune response, that is capable of inhibiting distal tumors outside the irradiation field.
Interestingly, radiotherapy is known to enhance and inhibit the immune response. For example, irradiated tumor cells release tumor neoantigens, which lead to tumor killing via activated T cells. What’s more, effector T cells can migrate to distant tumor sites to induce the abscopal effect. But radiotherapy can also result in tumor immune escape by upregulating immune checkpoints that inhibit T cell activation and function (see image below).
Mechanisms through which radiation enhances and inhibits the immune response.
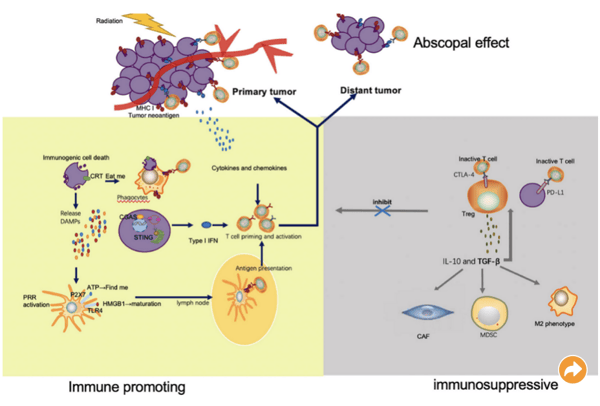
Image from Meng et al. (2021). The Combination of Radiotherapy with Immunotherapy and Potential Predictive Biomarkers for Treatment of Non-Small Cell Lung Cancer Patients. Frontiers in Immunology. 21 September 2021; doi.org/10.3389/fimmu.2021.723609. Used under Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
The additive or synergistic effects of radiotherapy and immunotherapy are well known and have been shown to enhance the abscopal effect (see here for an example). Still, the sequence of radiation therapy and immunotherapy administration remains imprecise, though this will likely change in the near future since there are currently 70+ clinical trials investigating combination radiotherapy with CTLA-4 or PD-1 blockade in many different tumor types, radiation doses, and timing and sequence of checkpoint blockade.
Understanding the immune mechanisms, which drive abscopal responses, is critical to designing treatment regimens that improve the joint potential of irradiation and immunotherapy. Preclinical models build a better understanding of the immune mechanisms that drive abscopal effects and will help to develop novel combination treatment regimens as a result. To enhance the translatability of preclinical studies of combination therapy, researchers need to select the right preclinical models and study designs first.
Using Preclinical Models to Support Radiotherapy and Immunotherapy Combinations
The sequence of administration of anti-PD-1 therapy and radiotherapy determines the magnitude of the abscopal response. When anti-PD-1 therapy is administered before radiation therapy, tumor antigen-specific CD8+ T cell expansion is delayed and the abscopal effect of combined therapy is lost. But when anti-PD-1 therapy is administered after radiation therapy, activated T cells (especially CD8+ T cells) proliferate, recognize the tumor, and exert a potent killing effect.
Preclinical platforms with validated and characterized mouse immuno-oncology models, such as well-characterized subcutaneous syngeneic tumor models, are immensely helpful when selecting a model to investigate combined irradiation and immunotherapy regimens. In addition to syngeneic tumor models, cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) models can also be used in immunodeficient or humanized models (reconstituted with CD34+ HSC or PBMCs).
These models can then be combined with X-ray-based focal beam radiation using an instrument such as the Xrad SmART irradiator that allows the delivery of high radiation doses (e.g., 20+ Gy) to subcutaneous tumors and well-defined orthotopic tumors, with minimal surrounding tissue toxicity. This irradiator also combines CT imaging, which allows software-based design/positioning of treatment beams, often a better option for high radiation doses and orthotopic tumors close to sensitive tissues such as the brain.
By pairing the appropriate in vivo model with a precise irradiator, researchers can investigate immunotherapy potentiation (e.g., checkpoint inhibitor combinations) and radiosensitizers.
In addition, in vitro irradiation studies are commonly conducted using cell lines or tumor organoids, such as PDX organoid (PDXO) or patient-derived organoid (PDO) models in combination with chemotherapy or immunotherapy alone or using immune cell/organoid co-cultures.
Using the right preclinical models for your specific research questions can go a long way in helping to make sure your novel treatment regimen is positioned for success.
Conclusion
Combination strategies of irradiation and immunotherapy harness the immune system to improve responses in difficult-to-treat and/or unresponsive tumors. But, determining the administration sequence of radiotherapy and immunotherapy is not straightforward, but important for maximizing the potential of combination therapy regimens.
We encourage researchers to consider your models and study designs before beginning preclinical studies for combination radiotherapy and immunotherapy treatment regimens. Our highly skilled and experienced scientists can help you optimize your study protocols so that your study has the best chances of success.
Visit our dedicated immuno-oncology site to learn more about how we are helping researchers progress their immuno-oncology treatment regimens.