 We presented a wide range of oncology modelling capabilities at AACR 2018, spanning the breadth of preclinical research. Four of our posters addressed developments in vitro and ex vivo assay platforms; this blog post looks at the new data presented on an IDO1 compound screening and testing platform, an in vitro assay platform to test anti-CD47 agents, and ex vivo PDX platforms for 3D compound testing and screening and to assess novel agents targeting the tumor microenvironment.
We presented a wide range of oncology modelling capabilities at AACR 2018, spanning the breadth of preclinical research. Four of our posters addressed developments in vitro and ex vivo assay platforms; this blog post looks at the new data presented on an IDO1 compound screening and testing platform, an in vitro assay platform to test anti-CD47 agents, and ex vivo PDX platforms for 3D compound testing and screening and to assess novel agents targeting the tumor microenvironment.
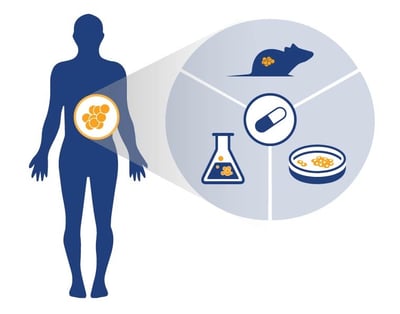
AACR 2018 Poster 3809: T Cell Activation through the Inhibition of Tumor-Expressed IDO1 Activity in the Tryptophan Metabolism Pathway
IDO1 inhibitors have been in the news recently, due to the Phase III failure of epacadostat (IDO1 inhibitor) in combination with Keytruda® (PD-1 inhibitor). Many in the bioscience community are discussing what this means for the class of drugs as a whole, and this poster was our contribution to that conversation.
 Assessing IDO1 Inhibitors In Vitro
Assessing IDO1 Inhibitors In Vitro
IDO1 is an important immuno-oncology drug development target due to its suppression of T cell function. This suppression occurs when tryptophan is depleted, due to its breakdown by IDO1. Targeting this IDO1-mediated tryptophan metabolism pathway (to stop the breakdown of tryptophan) should rescue T cell function, with many IDO1-targeting agents now in development.
To assess the activity of these compounds, we’ve developed an agent screening and testing platform. This in vitro assay system assesses the amount of a tryptophan breakdown product (N-formyl-kynurenine, Kyn) in culture medium, plasma, and tumor tissues as a measure of IDO1 activity.
Initial IDO Compound Screening and Testing Platform
The poster runs through a range of features of the platform, including the initial ability to accurately measure IDO1 gene expression in cell lines (either constitutive or induced expression), and that the platform produces expected cell proliferation curves for cell lines treated with cisplatin and an IDO1 inhibitor.
The assay is shown to effectively measure inhibition of IDO1 through Kyn levels assayed by LC-MS/MS, and to be more effective at doing so when there is a longer compound treatment window. In vitro and in vivo inhibition of IDO1 is also studied and presented.
To summarize, this poster details an in vitro assay platform which shows the reliable detection of IDO1 activity through Kyn measurement, when IDO1 is inhibited via treatment with targeted inhibitors alone and in combination with other targeted immunotherapy.
AACR 2018 Poster 4556: Phagocytosis Response of Macrophages to the CD47 Expression of Tumor Cells in Anti-CD47 Therapy
 CD47 is a transmembrane protein that belongs to the immunoglobulin family. It acts as a “don’t eat me” signal, inhibiting cell clearance by macrophages. Many cancer cells work around this by overexpressing CD47; therefore, blocking the protein with specific targeted antibodies should restore the phagocytic, antitumor activity of macrophages.
CD47 is a transmembrane protein that belongs to the immunoglobulin family. It acts as a “don’t eat me” signal, inhibiting cell clearance by macrophages. Many cancer cells work around this by overexpressing CD47; therefore, blocking the protein with specific targeted antibodies should restore the phagocytic, antitumor activity of macrophages.
This poster details a robust in vitro system to test anti-CD47 agents. The assay assesses the phagocytic activity of macrophages that have been exposed to tumor cells and to therapeutic agents targeting CD47.
Assessing CD47 Targeting Agents via Macrophage Antitumor Activity
After detailing the key assay methods, the poster goes on to identify tumor cells lines expressing high levels of CD47 via FACS, with Karpas299 and SK-OV-3 cells lines being the chosen cell lines. While SK-OV-3 is a frequently used model in phagocytosis studies, Karpas299 actually expresses more CD47, inducing a more robust target engulfing activity and providing a better model to test anti-CD47 antibodies.
The levels of M1 and M2 polarized macrophages in Karpas299 tumors were studied, with FACS data showing that administration of anti-CD47 restores phagocytosis, as measured by the level of CD14+ CFSE+ double positive cells. M1 and M2 macrophages demonstrate comparable anti-CD47 antibody-specific phagocytic activities, whereas anti-CD274 (PD-L1) treatment did not yield phagocytic activity any higher than the isotype controls.
Karpas299-hCD47 and SK-OV-3-hCD47 stable cell lines were also generated, and were used to establish strong positive correlation between phagocytic activity and CD47 expression level in the parental and the isogenic models.
Overall, this poster details a robust in vitro system to assess anti-CD47 targeting agents through the antitumor phagocytic activity of macrophages, and that disrupting the CD47-SIRPa anti-phagocytic axis is a novel immunotherapy approach against cancer.
AACR 2018 Poster 3861: 3D Ex Vivo PDX Cell Model Screening to Better Predict In Vivo Outcomes
 Patient-derived xenograft (PDX) models are widely used in preclinical drug development, due to their advantages over conventional xenograft models. These include:
Patient-derived xenograft (PDX) models are widely used in preclinical drug development, due to their advantages over conventional xenograft models. These include:
- Close molecular, histopathological, and heterogeneic reflection of the original tumor (more highly conserved than cell lines/GEMM).
- The correlation between PDX and clinical drug response profiles provides highly predictive preclinical data.
However, PDX models are not without their limitations; they are low throughput in candidate drug screening, have progressive loss of human-derived stromal elements over passages, and studies can be too time consuming and costly for large scale drug screening. To overcome these limitations, without sacrificing the advantages of PDX, we’ve developed a 3D PDX ex vivo assay platform for reliable and reproducible compound testing and screening.
Cost-Effective, Efficient Drug Screening and Efficacy Testing
We demonstrate that this assay platform can bypass these model limitations, using frozen tumor cells dissociated from freshly isolated, low passage PDX tumor tissues in 3D methylcellulose or soft agar assays for seven-day compound testing. Validation data confirms robust cell proliferation of PDX cells in the ex vivo 3D cell culture format, good cell viability, and data reproducibility across different passages of the same model.
Ex vivo data was compared with in vivo studies using the same models/treatments. Pearson Analysis showed >70% correlation, confirming the models maintain the correct drug response. The poster goes on to show how the assay can be used to profile panels of models, with varying potencies seen for four standard of care agents trialled across dozens of colorectal cancer models.
This platform is available for large-scale anticancer agent screening, combining the benefits of PDX models with a cost effective and efficient assay system.
AACR 2018 Poster 5033: Patient-Derived Xenograft Screening in a Three-Dimensional Tumor Growth Assay incorporating Stromal Elements to Recapitulate the Human Tumor Microenvironment
 The assessment of novel tumor microenvironment (TME) targeting therapies is currently hampered by a lack of preclinical in vitro and in vivo models which contain, or incorporate, human stromal cells.
The assessment of novel tumor microenvironment (TME) targeting therapies is currently hampered by a lack of preclinical in vitro and in vivo models which contain, or incorporate, human stromal cells.
This poster details the development and use of a novel 3D-tumor growth assay (3D-TGA) which meets this need by providing a platform of patient-derived tumor cells admixed with a basement membrane extract and human bone marrow-derived mesenchymal stem cells (bmMSCs) in a 96-well format for compound screening.
Clinically Relevant Ex Vivo Platform for Efficacy Screening
After detailing the assay methods, we looked at the assessment of four NSCLC PDX models in this platform. Firstly, the efficacy of paclitaxel plus an mTOR inhibitor is compared for an in vivo study and in this ex vivo assay. Similar efficacy was observed in the 3D-TGA and NSCLC PDX models; however, a combination finding seen in vivo was not observed in the 3D-TGA unless bmMSCs were included, demonstrating the importance of stromal elements for in vivo recapitulation.
The relative potency of standard of care agents between models was tested using the panel of NSCLC models/cells. Results from the 3D-TGA incorporating stromal elements was shown to correlate to in vivo efficacy data across several NSCLC PDX models, including unique models with clinically relevant mutations.
In summary, the 3D-TGA incorporating PDX tumor cells and stromal elements is a clinically relevant platform that accurately recapitulate the in vivo tumor environment for reliable in ex vivo efficacy screening.
Watch out for our other blog post summaries to catch up with all the latest AACR 2018 data on humanized models, murine tumor homograft models, PDX models, as well as microbiome studies.